In this piece, let’s discuss the recent positive events that have unfolded for Global Blood Therapeutics.
By Alexander J. Poulos
Global Blood Therapeutics
In our initial coverage of Global blood Therapeutics (GBT), we presented our bullish thesis based on some exciting phase 2 data for GBT 440 for the treatment of Sickle-Cell Disease (SCD). We believe the most salient point from our initial report “Best in Biotech? Global Blood Therapeutics’ Promising Treatment for Sickle Cell Anemia”
We believe the dramatic drop in irreversibly sickled cells bodes well for the ultimate commercial potential of the product in our opinion. The stunning drop in sickled cells of 77% at the median versus a 9.7% increase in the placebo arm indicates, in our view, the product is highly effective thus far. Based on the initial data reads, the FDA has designated GBT440 Fast Track, Orphan Drug, and Rare Pediatric Designations. The Rare Pediatric designation is particularly valuable as viable options for children remains scarce. The European Medicines Agency added GBT440 in its Priority Medicines (PRIME) program and declared GBT440 an orphan medicinal product for the treatment of patients with SCD. While designation is not an assurance of commercial success, we are comforted by the FDA’s and its European counterpart’s designations as it enhances the product’s potential for approval–as regulators are keenly interested in accelerating the product’s path to market (due to a significant unmet need). Assuming final testing parallels the results shown thus far, we feel a clear path to market is established.
Quote Source: Valuentum Securities
The key for Global is to replicate the impressive results in a much-larger phase 3 setting. We would like to re-emphasize the importance of the clinical data; if GBT 440 (now named Voxelotor) does not reproduce the initial findings the vast majority of the value of an equity stake in Global Blood Therapeutics would immediately vanish. With the above warning in mind, we have been pleased with the recent data release detailing the results achieved with a 67-year-old male patient that is afflicted with SCD. In this particular case the patient is far older than the typical SCD patient. To further complicate matters, the patient has developed red blood cell antibodies from multiple blood transfusions which prevent further treatment for his anemia (a side effect of SCD).
After receiving Voxelotor 900 mg orally once daily, the patient showed a rapid response (in 1 to 2 weeks), with an improvement in pain, fatigue, and overall mental health (as measured by the Patient Health Quality-9 score). His hemoglobin levels rose quickly to approximately 1.5 g/dL above baseline with a sustained increase over 66 weeks in a range of 1 to 1.5 g/dL. Reductions occurred in reticulocyte count (an indicator of increased production to replace damaged red blood cells) and bilirubin (a measure of red blood cell destruction), both consistent with diminished hemolysis. Blood oxygen saturation improved on standard walk test, from 86mmHg at baseline to 96mmHg at 65 weeks, and he discontinued continuous oxygen supplementation. No hospitalization prompted by sickle cell pain has occurred since Voxelotor initiation. His sole treatment-related side effect, Grade 2 diarrhea, occurred nine weeks after starting voxelotor treatment when the dose was increased to 1,500 mg daily, but resolved upon return to 900 mg with no further treatment-related side effects. Clinical and laboratory improvements have continued for more than 17 months, and he remains on treatment today under compassionate use access.
Quote Source: Global Blood Therapeutics
The results posted are nothing short of spectacular, though we would like to caution the results are from only one patient in a compassionate-use setting. Nevertheless, the results posted for an immediate reversal in hemolysis leading to a sharp spike in Hemoglobin levels seen in phase 2 testing is evident in this case as well. We found the discontinuation of oxygen therapy as particularly noteworthy as the new freedom enjoyed by the patient demonstrates the tremendous improvement in the quality of life for the patient.
Safety Review
Voxelotor is in the midst of a rigorous phase 3 study with an expected read out in the first half of 2019. Global has scheduled an independent safety review to analyze the data thus far in the trial to determine if the trial should proceed as planned. Voxelotor was approved for continued study as there are no safety signals or discontinuation of therapy thus far due to side effects. Again, we would like to emphasize that, even though we are pleased with the results, there are no guarantees of success.
The safety review also analyzed the results of the phase 2 HOPE-KIDS 1 study. The results are in-line with what was seen in the adult trials. In what may be a market moving event, Global will release initial phase 2 HOPE-KIDS data at the upcoming American Society of Hematology Annual Meeting in December.
Volatility
The share price of Global Blood Therapeutics can be volatile with aggressive intra-day moves. We believe speculators have entered the market as the equity has posted a sharp move higher during the past couple of months. We would like to share some probability data from the Biotechnology Innovation Organization that was collected from 2006-2015. The area with the highest phase 3 success is the hematology market (hematology is the study of diseases of the blood—SCD falls into this category with Oncology posting the worst results with a paltry 40% success rate.
While past results are no guarantee of future results, if past precedent holds, the odds are generally favorable of success in phase 3 for Global Blood Therapeutics. We think providing a clear overall picture of the inherent risks involved so investors can make fully informed, educated decisions in their investments only makes sense, so gauging the probabilities of success of phase 3 is well worth knowing, as outlined in the table below.
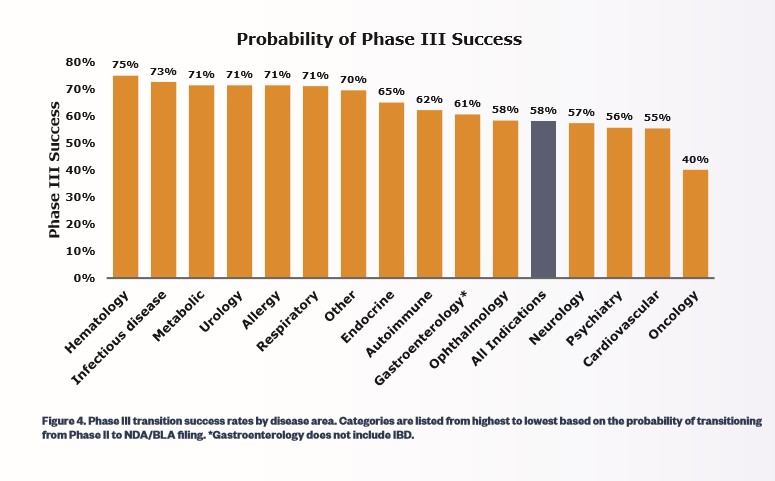
Image Source: Biotechnology Innovation Organization
Conclusion
Though we note that an investment in any biotech equity has tremendous risk of financial loss, something that should be well-understood, we remain intrigued by Global Blood Therapeutics as the data continues to underpin the excellent science behind Voxelotor. If Global is indeed successful in bringing Voxelotor to market, we believe it may become the de facto treatment of choice; the elegant once-a-day oral dosage forms thus far have a rather minimal side effect profile, a remarkable breakthrough from where we stand. Many have pointed to gene editing, but we feel the cost will far outweigh the benefits, especially when compared to a one day dose. We will continue to monitor events as they unfold with timely updates when warranted.
Independent Biotech Contributor Alexander J. Poulos is long Global Blood Therapeutics.
