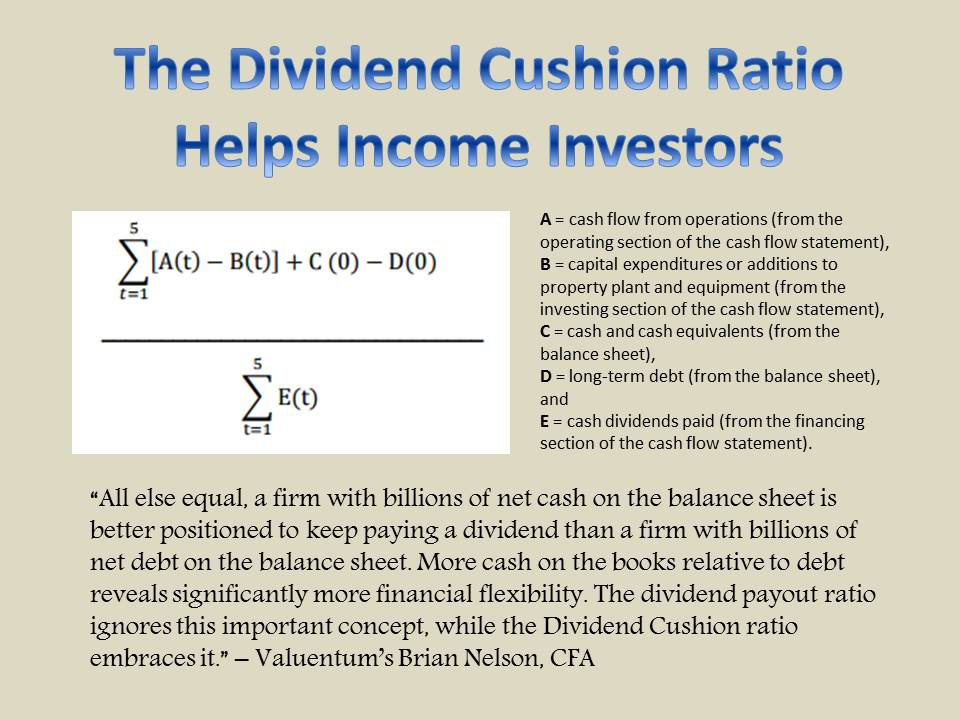
Member LoginDividend CushionValue Trap |
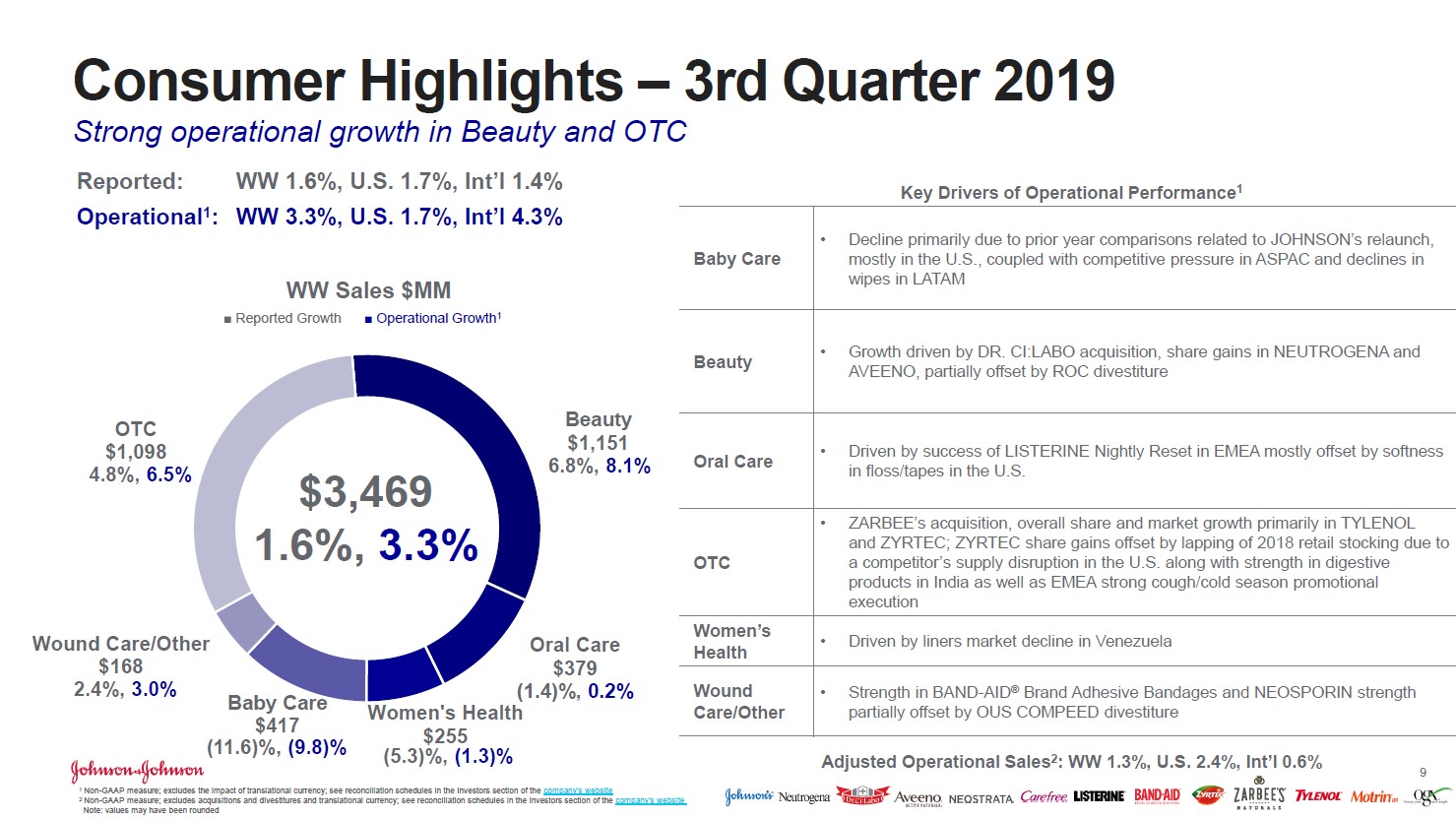
Johnson & Johnson’s Talc Problems Hit Another Bump
To gain access to the members only content, click here to subscribe. You will be given immediate access to premium content on the site.
|