Image Source: TradingView
By Brian Nelson, CFA
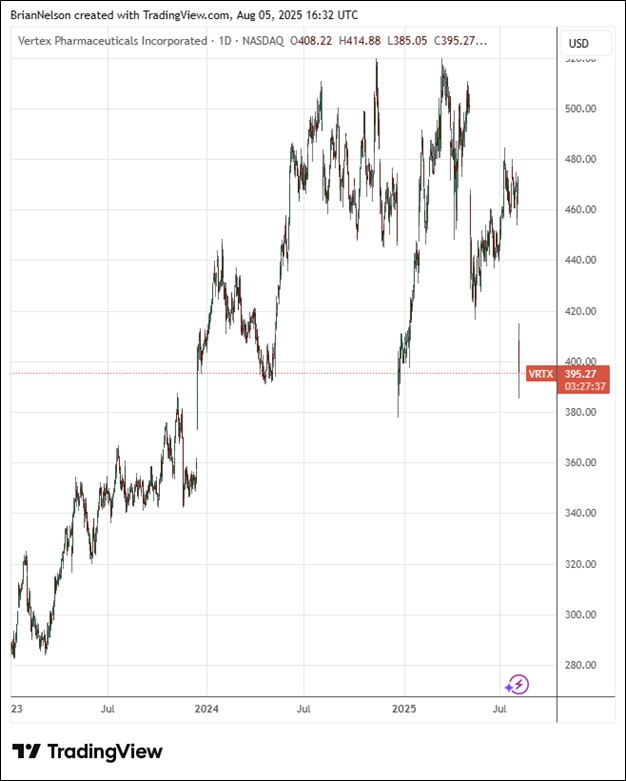
On August 4, Vertex Pharma (VRTX) reported strong second quarter results with both revenue and non-GAAP earnings per share coming in better than expected. The solid performance, however, was overshadowed by a disappointment in its pipeline for pain management:
Vertex announced results from its Phase 2 placebo-controlled dose-ranging study evaluating the safety and efficacy of VX-993, an investigational selective NaV1.8 pain signal inhibitor, in treating acute pain after bunionectomy surgery. Treatment with VX-993 did not result in a statistically significant improvement on the primary endpoint of the time-weighted sum of the pain intensity difference from 0 to 48 hours (SPID48) compared to placebo. VX-993 was generally safe and well-tolerated. Based on these results, Vertex will not further advance VX-993 as monotherapy in acute pain.
Despite the setback, Vertex’s second quarter results were actually quite good. Total revenue increased 12%, to $2.96 billion, beating consensus by $50 million, thanks to continued performance of its cystic fibrosis therapies and early contributions from ongoing launches (ALYFTREK in cystic fibrosis, JOURNAVX in acute pain, and CASGEVY for sickle cell disease and beta thalassemia).
In the U.S., total revenue increased 14%, to $1.85 billion due in part to continued strong patient demand. Outside the U.S. total revenue increased 8%, to $1.12 billion. GAAP and non-GAAP net income were $1 billion and $1.2 billion, respectively, compared to net losses in the prior period given the impact of AIPR&D expense resulting from its acquisition of Alpine Immune Sciences.
Vertex reiterated its full year 2025 guidance calling for revenue in the range of $11.85-$12 billion, which assumes continued growth in its cystic fibrosis franchise, “including the global launch of ALYFTREK, continued uptake of CASGEVY in multiple regions, and early contributions from the U.S. launch of JOURNAVX.” The company reiterated its guidance for R&D, AIPR&D, and SG&A expenses and its non-GAAP effective tax rate guidance of 20.5-21.5%. The guidance includes an immaterial cost impact from tariffs in 2025.
Though the trial setback in pain management has sent shares of Vertex Pharma tumbling, we continue to like Vertex’s net cash rich balance sheet, cystic fibrosis franchise, its groundbreaking CRISPR/Cas9 gene-edited therapy and its novel non-opioid pain medicine. Shares remain a holding in the Best Ideas Newsletter portfolio. Our $445 per share fair value estimate remains unchanged at this time.
—–

Brian Nelson owns shares in SPY, SCHG, QQQ, QQQM, DIA, VOT, RSP, and IWM. Valuentum owns SPY, SCHG, QQQ, QQQM, VOO, and DIA. Brian Nelson’s household owns shares in HON, DIS, HAS, NKE, DIA, RSP, SCHG, QQQ, QQQM, and VOO. Some of the other securities written about in this article may be included in Valuentum’s simulated newsletter portfolios. Contact Valuentum for more information about its editorial policies.
Valuentum members have access to our 16-page stock reports, Valuentum Buying Index ratings, Dividend Cushion ratios, fair value estimates and ranges, dividend reports and more. Not a member? Subscribe today. The first 14 days are free.
