
Image Source: JNJ third quarter earnings presentation
Shares of newsletter portfolio holding Johnson & Johnson continue their stellar run, posting new all-time highs on the heels of an impressive third quarter earnings report. Let’s take an in-depth review of key pharma product franchises as they relate to the performance of the stock.
By Alexander J. Poulos and Kris Rosemann
Immunology
Johnson & Johnson’s (JNJ) largest pharmaceutical division remains Immunology–an area where the company continues to be a pioneer–but the division has begun to feel the strain of the coming biosimilar onslaught as its legacy molecule Remicade continues to cede ground to biosimilar competition. The drug posted a 7.6% year-over-year revenue decline in the third quarter of 2017 to ~$1.65 billion as biosimilars continue to take share and essentially place a cap on potential price hikes.
Fortunately for J&J, the continued growth in Stelara more than offset the weakness in Remicade, but the growth trajectory of Stelara may not be secure going forward. The recent crop of biologic agents such as Talz, Kevzara, and Cosentyx, in addition to the expected growth of the Jak-1 class, will likely pressure growth of Stelara. To J&J’s credit, however, it was able to expand the prescribing label for Stelara to include Crohn’s Disease, where strong uptake was a driver of growth in the third quarter of 2017.
We expect the Immunology division to remain a nice profit driver, but the group’s best days may be behind it. J&J is in the process of bringing to market two new molecules in an attempt to maintain market share in Immunology. The first of the two, Sirukamab, a product for Rheumatoid Arthritis, received a complete response letter (CRL) requesting additional clinical data to determine the side effect profile of the product. Such a development will inevitably push back a potential launch of the product.
However, the company did receive a dose of good news with the approval of Tremfya for the treatment of plaque psoriasis. Tremfya will enter a crowded field of next-generation products, but Tremfya performed splendidly in head-to-head against market leader Humira. Tremfya’s outstanding results compared to Humira should be exploited by J&J in gaining favorable formulary placement.
Oncology
We remain impressed with the growth trajectory of Johnson & Johnson’s Oncology division as it posted 25% year-over-year revenue growth in the third quarter of 2017–the kind of growth typically seen from a junior biotech. Its top-selling product remains Zytiga, a treatment for prostate cancer, but Zytiga’s future growth trajectory is in doubt as the molecule faces significant patent uncertainty.
Zytiga posted sales north of $2 billion in 2016, a worthy target for the generic drug industry. Zytiga’s 213 patent (which expired in December 2016) is the original composition of matter patent, while its 438 patent (the patent currently in question) was granted much later in the life cycle of the product, which could make the product’s patent protection more vulnerable. Patent challenges are notoriously challenging to handicap, and even if J&J loses the case, the loss of sales will likely be masked by the company’s overall revenue of more than $70 billion. However, such a setback would impact the growth trajectory of the Oncology division, which could have bigger than expected ramifications on the firm’s share price as Oncology continues to be an area of emphasis across pharma.
Perhaps the greatest strength of Johnson & Johnson is its broad product diversity that can offset the potential loss of even a $2 billion revenue product, and continued growth of drugs such as Darzalex and Imbruvica are sources of confidence for investors. Darzalex is an innovative new treatment for multiple myeloma and remains firmly entrenched in the early stages of its growth trajectory, and we expect J&J to broaden the prescribing label for the treatment, thus enhancing its sales trajectory in coming years. Imbruvia, on the other hand continues its torrid run of growth as it remains relatively unchallenged in lymphoma. Gilead Sciences’ (GILD) Zydelig remains a very distant second in overall prescriptions written and new perscriptions written.
Cardiovascular/Metabolism
Results from Johnson & Johnson’s Cardiovascular division have begun to demonstrate the strain of recent negative clinical developments, though it was able to continue delivering year-over-year revenue growth of 3%+ in the third quarter of 2017. Revenue erosion of Invokana, the company’s formerly dominant subtype 2 sodium-glucose transport inhibitors (SGLT-2), has begun in what may be an irreversible decline. Unfortunately for J&J, Invokana posted a slightly increased risk of amputations, an area of notable concern for diabetics. The results may very well be the death knell for the product with recent prescription data confirming bearish sentiment surrounding the drug. Eli Lilly’s Jardiance is now in the cat-bird seat with a noticeable ramp in new prescriptions written, and we expect Jardiance to become the king of the class, depriving J&J of a once promising asset and market position.
The news on the Xarelto front for Johnson & Johnson is also a bit of a mixed bag with clinical data coming in less robust than expected, and the firm needs additional positive clinical data to prevent the ceding of further ground to Eliquis. Xarelto posted an impressive 20% jump in quarterly sales to $635 million in the third quarter of 2017, but it is no longer the top-seller in the field. Eliquis also has a more robust prescribing label, which could allow it to negotiate more favorable formulary placement and deprive Xarelto of sales moving forward. However, management has recognized its vulnerability and is working to shore up this area of potential weakness. The recent acquisition of Actelion was likely consummated with an eye towards melding the Pulmonary Arterial Hypertension products with J&J’s Cardio unit to mask the weakness of Xarelto and Invokana.
Clinical Pipeline
Johnson & Johnson has suffered two late stage setbacks with molecules it initially expected to help serve as catalysts for its next leg higher. The first molecule is the previously-mentioned Sirukumab, an anti-interleukin-6 antibody for the treatment of rheumatoid arthritis. These developments could represent a missed opportunity for J&J as the company has withdrawn its applications for Sirukumab in RA in light of the less than stellar safety data. Aiding in the decision is the competitive landscape with Roche’s (RHHBY) Actemra and Regeneron (REGN) and Sanofi’s (SNY) Kevzara collaboration–both of which have delivered safety data far superior to that of Sirukumab. As a point of reference, Actemra generated sales of ~1.4 billion Swiss Francs in the first nine months of 2017 as the product is well into its life cycle after gaining initial approval in 2010.
The recent string of pipeline disappointments extend into Johnson & Johnson’s Oncology division after the company terminated the development of Talacotuzumab for the treatment of acute myeloid leukemia (AML). J&J moved the product into phase 3 testing in early 2017 but quickly reversed field as the safety data remained unappealing. Such a change of course underscores the inherently risky nature of new drug discovery, and even large cap behemoths such as J&J can fall victim to the spell of clinical data.
From our point of view, the Actelion acquisition is management’s attempt at masking the weakness of the Cardiovascular division and restoring its pipeline with assets from Actelion’s. J&J is faced with the unenviable task of having to replace the revenue stream of its top-selling product (Remicade), while suffering a setback to the growth rate of a key asset (Invokana), which will place additional strain on the high-growth Oncology division to make up the loss of expected revenue.
Conclusion
We expect to continue to include Johnson & Johnson in both newsletter portfolios for the foreseeable future. The firm’s balance sheet health is not what it once was after the Actelion acquisitions–it holds net debt of ~$19 billion as of the third quarter of 2017–but we continue to expect robust free cash flow generation to be sufficient in delivering a competitive dividend. The company has yet to submit its 10-Q for the third quarter of the year, but through the first six months of 2017, free cash flow came in at more than $7.4 billion, up from just under $5.4 billion in the same period of 2016 and far higher than cash dividends paid of ~$4.4 billion. Its Dividend Cushion ratio is currently a strong 1.9 to go along with a yield of ~2.4% at recent price levels.
Following a solid third quarter, Johnson & Johnson raised its reported sales guidance for 2017, as well as its adjusted EPS guidance. Operational sales growth guidance remains unchanged at 5.5%-6%, but reported sales growth guidance has been increased to 6%-6.5% due to movement in the EUR/USD exchange rate. Adjusted EPS guidance on an operational and constant currency basis has been increased to a range of $7.22-$7.27, up from previous guidance of $7.17-$7.27 and initial guidance of $7.05-$7.20. In its quarterly conference call, management also reiterated its expectations for above market growth in its Pharmaceutical segment through 2021 despite recent setbacks in some key pipeline assets. If the company is able to deliver on such an initiative, it would achieve its goal of delivering a decade of revenue CAGR above the branded pharma market.
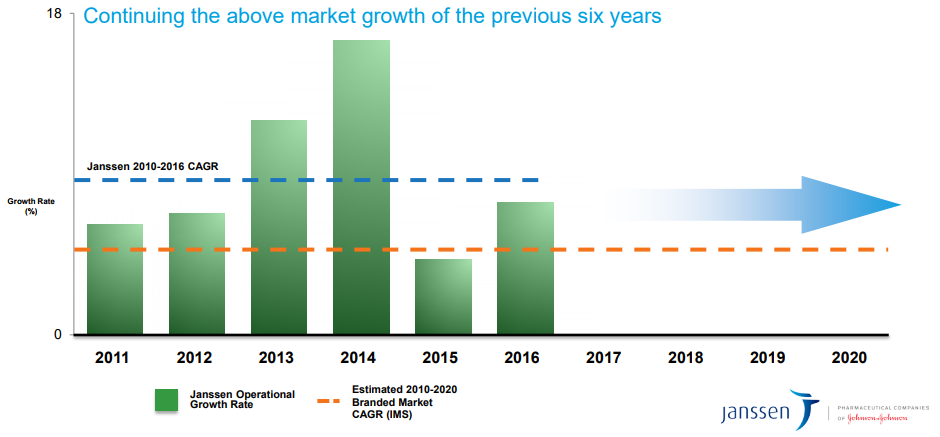
Image source: JNJ Pharma Overview Presentation
Johnson & Johnson has been a tremendous performer in the Best Ideas Newsletter portfolio since it was added at ~$104 per share in January 2016, and the company is the largest weighting in our Dividend Growth Newsletter portfolio, where it has been a holding since the portfolio’s inception. Shares have experienced quite the run of late and are bumping up against the upper bound of our fair value range, but the Valuentum strategy often includes “letting winners run” as long as technical momentum remains supportive of strong fundamentals. Such appears to be the case with Johnson & Johnson, and we continue to like the unique diversity of its business model, which combines its strong pharma portfolio with the steady characteristics of its Consumer segment and the growth potential of its Medical Devices segment.
Disclosures: Independent Healthcare and Biotech Contributor Alexander J. Poulos is long Regeneron Pharmaceuticals and Gilead Sciences.
