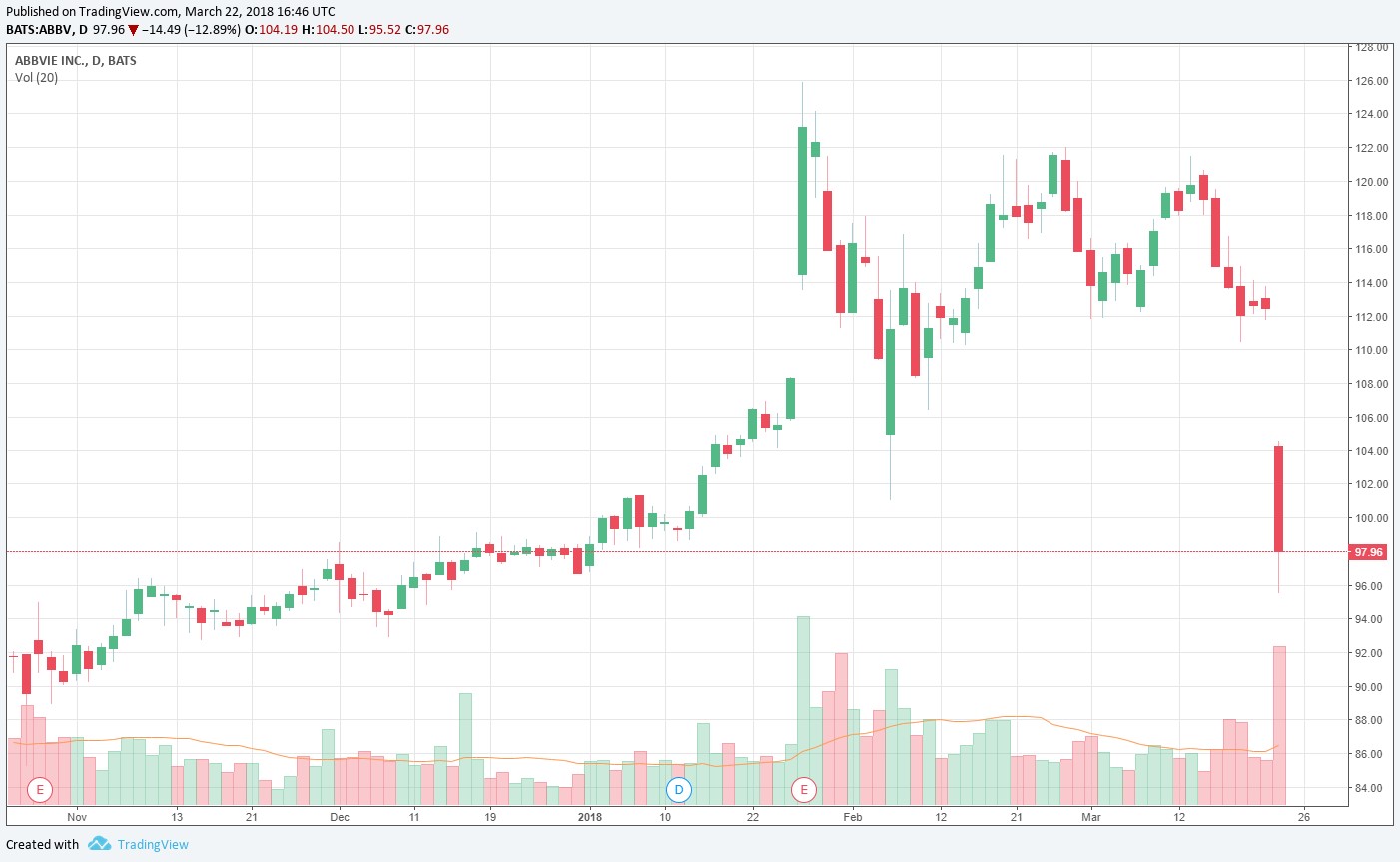
AbbVie will not seek accelerated approval for what had been expected to be its next blockbuster drug, Rova-T, which had been investigated for third-line relapsed/refractory small cell lung cancer. The market is reacting negatively to the disappointment.
By Brian Nelson, CFA
Hopes had been high for the performance of a phase II study of AbbVie’s (ABBV) Rova-T (Rovalpituzumab Tesirine), an antibody conjugate targeting cancer stem cell-associated delta-like protein 3 (DLL3), but the results of the study “were not what (management) hoped for.” The company noted that, after consulting with the FDA, “it will not seek accelerated approval for Rova-T.” AbbVie’s stock was hammered on the disappointing news.
Though AbbVie will continue to evaluate Rova-T in first and second-line small cell lung cancer, the market was betting that Rova-T (given that DLL3 is found in 80% of small cell lung cancer patient tumors) would be a blockbuster drug in AbbVie’s pipeline that would help it slowly diversify away from Humira, which currently accounts for the majority of the company’s revenue and profits. With the disappointing study findings for Rova-T for third-line relapsed/refractory small lung cancer, however, it appears AbbVie’s concentration risk with Humira will not be going away anytime soon.
We had not been building in much of anything with respect to Rova-T in our valuation model, so the company’s share-price collapse to the mid-$90s on the trading session March 22 could probably have been expected. AbbVie still has a number of things going for it, “Upadacitinib Posts Impressive Data for AbbVie (September 2017),” but as we noted in September, our cautious stance on shares remain. The company continues to rely on one product for the bulk of its revenue, and efforts to pursue diversification and replacement products are not guaranteed to bear fruit. Unlike a going-concern, branded drugs have finite lives until generics enter the fray.
That said, we continue to like AbbVie’s prospects with Imbruvica (now Orphan Drug status here) and Upadacitinib (updated study here), as we mentioned last September, but neither opportunity may come close to generating the revenue it could lose as Humira’s dominance winds down. We think investors in AbbVie must have a strong stomach for risk because the company remains heavily exposed to shocks that could send shares tumbling 10%, 20% or more, mostly because of its lack of product diversification and the innate risk of any development pipeline. We’re viewing the company’s 35% increase to the dividend in February as a distraction for long-term holders, not an indication that things are looking up. Shares yield ~3.9% at the time of this writing, but we’re not biting.
—–
Valuentum members have access to our 16-page stock reports, Valuentum Buying Index ratings, Dividend Cushion ratios, fair value estimates and ranges, dividend reports and more. Not a member? Subscribe today. The first 14 days are free.
Brian Nelson does not own shares in any of the securities mentioned above. Some of the companies written about in this article may be included in Valuentum’s simulated newsletter portfolios. Contact Valuentum for more information about its editorial policies.
